Our website is not supported on this browser
The browser you are using (Internet Explorer) cannot display our content.
Please come back on a more recent browser to have the best experience possible
Today’s fourth industrial revolution is the transition from traditional manufacturing to large-scale automation via digital technologies, hence named Industry 4.0. This includes a vast umbrella of solutions and approaches such as:
The pharmaceutical industry’s transition to Industry 4.0, or Pharma 4.0, has been a long time coming, but it’s taking time for good reason. The industry faces aggressive production volumes and tight schedules on a daily basis. Vaccines and medicine must be produced around the globe without interruptions, so pharmaceutical companies cannot simply halt production to deploy a new solution.

Additionally, the core of pharma development requires much human oversight, making significant automation a challenge. That fact will not change because regulators must ensure the safety and effectiveness of those products.
However, the pharmaceutical industry has recently seen that digital transformation and automation can complement traditional pharma development instead of taking it over. Industry 4.0 principles can lead to more intelligent products and processes, streamline work, and improve product time-to-market. This also empowers employees to innovate and develop solutions, rather than watch over a manufacturing line.
Our client, a leader in the pharmaceutical industry, struggled to adopt Industry 4.0 technologies. Existing manual workflows and physical paperwork increased delays and were subject to a great deal of human error. Nearly 30% of the time, work was redone because of mistakes from manual, repetitive processes. They needed to implement a digital transformation program that would increase their production efficiency.
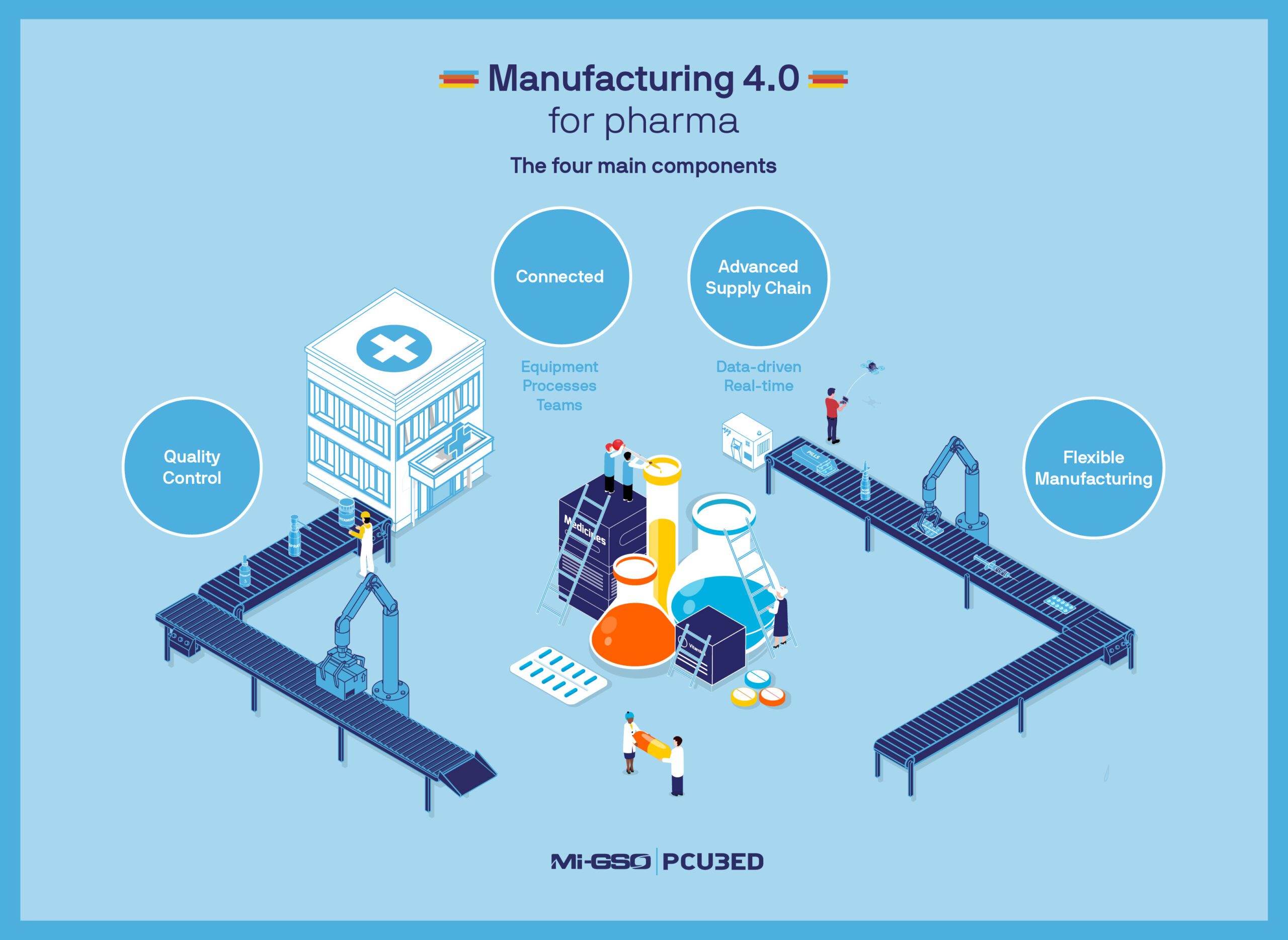
Executive leadership knew that digital tools, AI, and flexible manufacturing could make their processes far more efficient and adaptable. Even before our involvement, the client had already kicked off a portfolio of digital initiatives. This included many different process improvements and new products such as electronic batch records and deviation intelligence tools to monitor and reduce manufacturing inconsistencies.
To begin, the organization needed to gain support and alignment from all global regions. Each local site was ready to implement new technology but lacked a unified vision to develop their technology into a working product.
Additionally, the client faced aggressive production targets to deliver essential vaccines. Therefore, it was difficult to implement new technology without disrupting current manufacturing on the shop floor.
The client aimed to harmonize their Industry 4.0 deployment globally, pulling each of their separate work streams into a single transformation roadmap. This was where the MIGSO-PCUBED came in. We deployed our consultants across France, Canada, and the US to make sure the client’s digital solutions could be successfully implemented and would be sustainable with future growth.
Within one month, the MIGSO-PCUBED team deployed a global transformation team to lead a full-scope Transformation PMO, including:
The team developed three work streams for the program: one to establish the project and program delivery management, another to establish governance and collaboration, and the third to facilitate communication and change. Using these three workstreams, the PMO was able to provide full-scope transformation services supporting the continual adoption of the program.
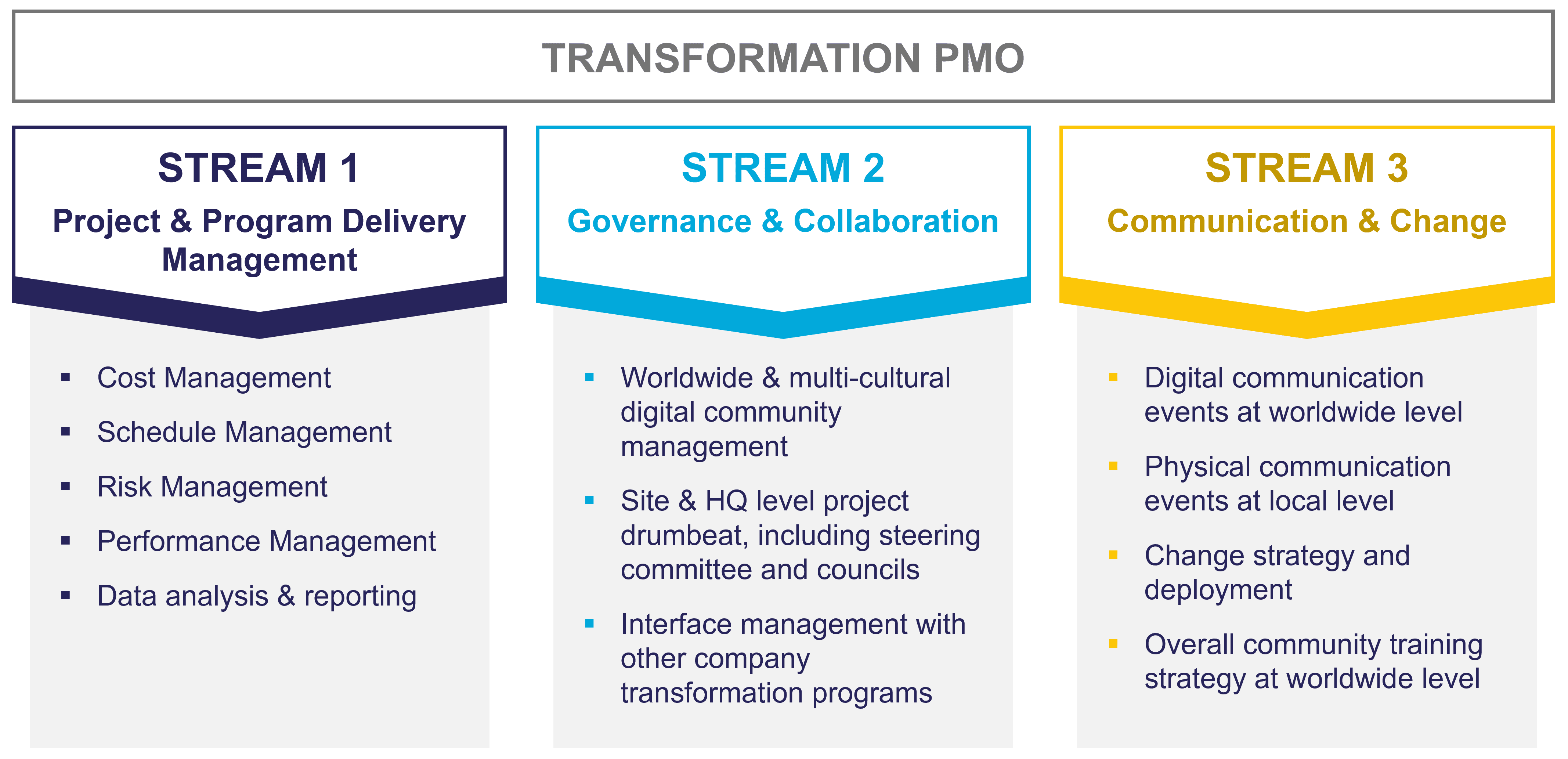
To deploy the client’s transformation program, we needed to set up the project controls and prepare the organization for change. That meant, we needed to establish the cost, schedule, risk, and performance management processes as well as a method for monitoring and reporting on these.
The cost and schedule management plans were important to set up first in order to set the timeline and key objectives for the program. One of the greatest challenges in doing so was aligning each stakeholder to determine the organization’s requirements and to set expectations that everyone could agree upon.
Another key component in securing successful program delivery was risk management. Every organization faces risk, but the impact of a global transformation program comes with additional risk both globally and locally. Our team helped the client to consider these risks early on and establish a process to manage them.
Finally, our team worked on developing a digital dashboard for the client to monitor KPIs and control progress throughout the transformation program. To date, our PMO team continues to manage the program’s core delivery and provide regular status updates for key stakeholders to take action before issues arise.
As we primed the teams for change, there was a clear need for effective governance processes. That meant structured communications with clear guidelines for all aspects of project management. Effective governance was crucial for managing the large-scale transformation because it would provide a viable structure for the organization to manage communications, risk, and future growth.
This was certainly the case for our client whose transformation program spanned several countries. Our team helped set up meeting cadences at both the site and global steering levels in order to establish collaboration across local and global teams.
Separately, MP also deployed a global agile delivery framework. This laid the foundation for a continuous delivery model focusing on high impact and value. Even after a few initial workshops, the organization saw greater commitment and alignment from their teams.

Change Management was one of the most crucial elements of the PMO. Not only did the client need to achieve global team alignment, but the program also had high-level visibility, reporting directly to the executive board.
MP started by developing a change strategy that would explain the program outcomes and establish consistent communications. Our consultants met with key stakeholders to discuss how the new technology would benefit their existing ways of working and drive adoption downstream. Our key messaging focused on how these digital solutions would:
Another key aspect of enabling change was training. It was important that all employees understood the new system and ways of working while feeling supported along the way. Our team developed a global training strategy and set up communication events, both physically and digitally, for it.
Even beyond the initial change, the training strategy was focused on upskilling the program teams. This way, employees could continue to develop their careers beyond the new and immediate program requirements. The strategy also incorporated a “train the trainer” approach so that the organization was well-positioned to onboard employees going forward.
With the help of the MP PMO team, our client clarified their digital transformation roadmap through 2025. By the end of 2021, the program office was on track to deliver all 215 of their KPI deliverables. They were also seeing the successful adoption of their change program. Additional benefits included shifting to more agile ways of working, implementing remote workflows, and decreasing plant cycle time by more than 25%.
Key to this has been the MP team’s ability to bring in insights from other industries around digital transformation best practices and governance.
Right away, worldwide governance and coordination were mobilized. Each regional site would report into a global MP PMO and a single client point of contact in France. Standardized templates across the sites, and the implementation of digital tools like Kanban boards and iObeya rooms, improved delivery within the portfolio of transformation initiatives.
Thanks to the successful adoption and delivery of their digital transformation program, the client is now able to better manage their digital products suite and is looking to extend their digital solutions beyond manufacturing plants. This enables them to develop products across the end-to-end value chain. For example, they will soon develop wearable technology and products for robotic assistance.
The client’s transition to Industry 4.0 is enabling their success for the future of the pharmaceutical industry and is allowing them to stay ahead of the market. We are excited to continue supporting them along this journey.
Loved what you just read?
Let's stay in touch.
No spam, only great things to read in our newsletter.
We combine our expertise with a fine knowledge of the industry to deliver high-value project management services.
MIGSO-PCUBED is part of the ALTEN group.
Find us around the world
Australia – Canada – France – Germany – Italy – Mexico – The Netherlands – Portugal – Romania – South East Asia – Spain – Switzerland – United Kingdom – United States
© 2024 MIGSO-PCUBED. All rights reserved | Legal information | Privacy Policy | Cookie Settings | Intranet
Perfect jobs also result from great environments : the team, its culture and energy.
So tell us more about you : who you are, your project, your ambitions,
and let’s find your next step together.
Dear candidates, please note that you will only be contacted via email from the following domain: migso-pcubed.com. Please remain vigilant and ensure that you interact exclusively with our official websites. The MIGSO-PCUBED Team
Choose your language
Our website is not supported on this browser
The browser you are using (Internet Explorer) cannot display our content.
Please come back on a more recent browser to have the best experience possible
